Microsoft word - ts 174.rtf
Allegato V - Modello per la Presentazione dei Programmi di Tirocini/Stage1 1. Soggetto Proponente (Operante in Sardegna) Ragione Sociale : Dipartimento Farmaco Chimico Tecnologico, Università degli Studi di Indirizzo : Via ospedale 72 – 09124 Cagliari Telefono: 070/6758571 Fax : 070/6758710 E-mail : [email protected] Rappresentante Legale : Prof. Anna Maria Fadda Referen
 Androgen responsiveness of L T2 cells · M A LAWSON and others 603
Although direct activation of AR leads to transcriptional
responses dependent on the interaction of ligand-bound AR with a competent promoter element, othermechanisms for AR-dependent activation of transcriptionhave been reported that are resistant to inhibition by theandrogen receptor antagonists 2-hydroxy-flutamide andcasodex (Peterziel et al. 1999). Therefore, to establish thatthe androgen responsiveness of pGL3-MMTV is depen-dent on the transcription activation properties of ligand-bound AR, we tested the ability of the AR antagonists2-hydroxy-flutamide and casodex to block the DHT-
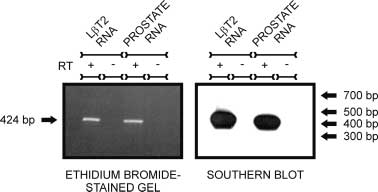
Figure 1 L T2 cells express AR mRNA. Primers specific for mouse
Androgen responsiveness of L T2 cells · M A LAWSON and others 603
Although direct activation of AR leads to transcriptional
responses dependent on the interaction of ligand-bound AR with a competent promoter element, othermechanisms for AR-dependent activation of transcriptionhave been reported that are resistant to inhibition by theandrogen receptor antagonists 2-hydroxy-flutamide andcasodex (Peterziel et al. 1999). Therefore, to establish thatthe androgen responsiveness of pGL3-MMTV is depen-dent on the transcription activation properties of ligand-bound AR, we tested the ability of the AR antagonists2-hydroxy-flutamide and casodex to block the DHT-
Figure 1 L T2 cells express AR mRNA. Primers specific for mouse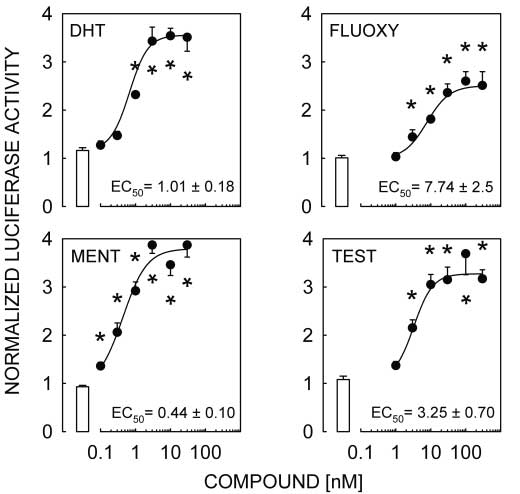 604 M A LAWSON and others · Androgen responsiveness of L T2 cells
Figure 2 DHT, FLUOXY, MENT and TEST activate the pGL3-MMTV reporter transfected
604 M A LAWSON and others · Androgen responsiveness of L T2 cells
Figure 2 DHT, FLUOXY, MENT and TEST activate the pGL3-MMTV reporter transfected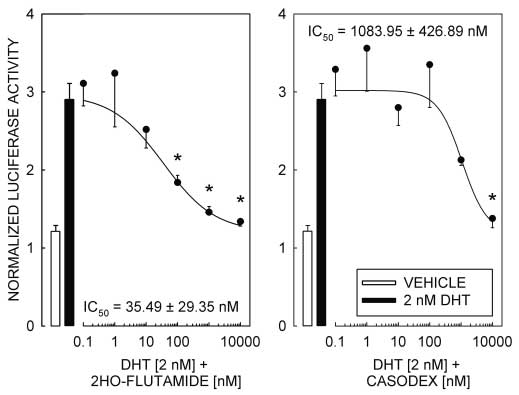 Androgen responsiveness of L T2 cells · M A LAWSON and others 605
Figure 3 AR antagonists 2-hydroxy-flutamide and casodex block DHT-induced reporter
Androgen responsiveness of L T2 cells · M A LAWSON and others 605
Figure 3 AR antagonists 2-hydroxy-flutamide and casodex block DHT-induced reporter