Lyme disease frequently asked questions (faq) - mn dept of health
Minnesota Department of Health Fact Sheet Lyme Disease Frequently Asked Questions (FAQ) How can I prevent myself from getting Lyme Check frequently for ticks, and remove them disease? Ticks actually have to bite you and remain Avoid possible tick habitats during the peak time of attached for one to two days before they can year (generally mid-May through mid-July). Blac

 Eects of the antibiotics oxytetracycline and tylosin on soil
Angel J. Baguera, John Jensenb,*, Paul Henning Kroghb
a Department of Toxicology, Faculty of Veterinary, University of Zaragoza, Miguel Servet, 177 E-50.013 Zaragoza, Spain
b Department of Terrestrial Ecology, National Environmental Research Institute, Vejlsùvej 25, P.O. Box 314, DK-8600 Silkeborg,
Antibiotics may enter the terrestrial environment when amending soils with manure. A Note of Guidance on
ecological risk assessment of veterinary medicines was issued in January 1998. Hardly any information about eco-
toxicological eects of already existing substances are available. This study has tested the eects of two widely used
antibiotics, tylosin and oxytetracycline, on three species of soil fauna: Earthworms, springtails and enchytraeids.
Eects of the antibiotics oxytetracycline and tylosin on soil
Angel J. Baguera, John Jensenb,*, Paul Henning Kroghb
a Department of Toxicology, Faculty of Veterinary, University of Zaragoza, Miguel Servet, 177 E-50.013 Zaragoza, Spain
b Department of Terrestrial Ecology, National Environmental Research Institute, Vejlsùvej 25, P.O. Box 314, DK-8600 Silkeborg,
Antibiotics may enter the terrestrial environment when amending soils with manure. A Note of Guidance on
ecological risk assessment of veterinary medicines was issued in January 1998. Hardly any information about eco-
toxicological eects of already existing substances are available. This study has tested the eects of two widely used
antibiotics, tylosin and oxytetracycline, on three species of soil fauna: Earthworms, springtails and enchytraeids.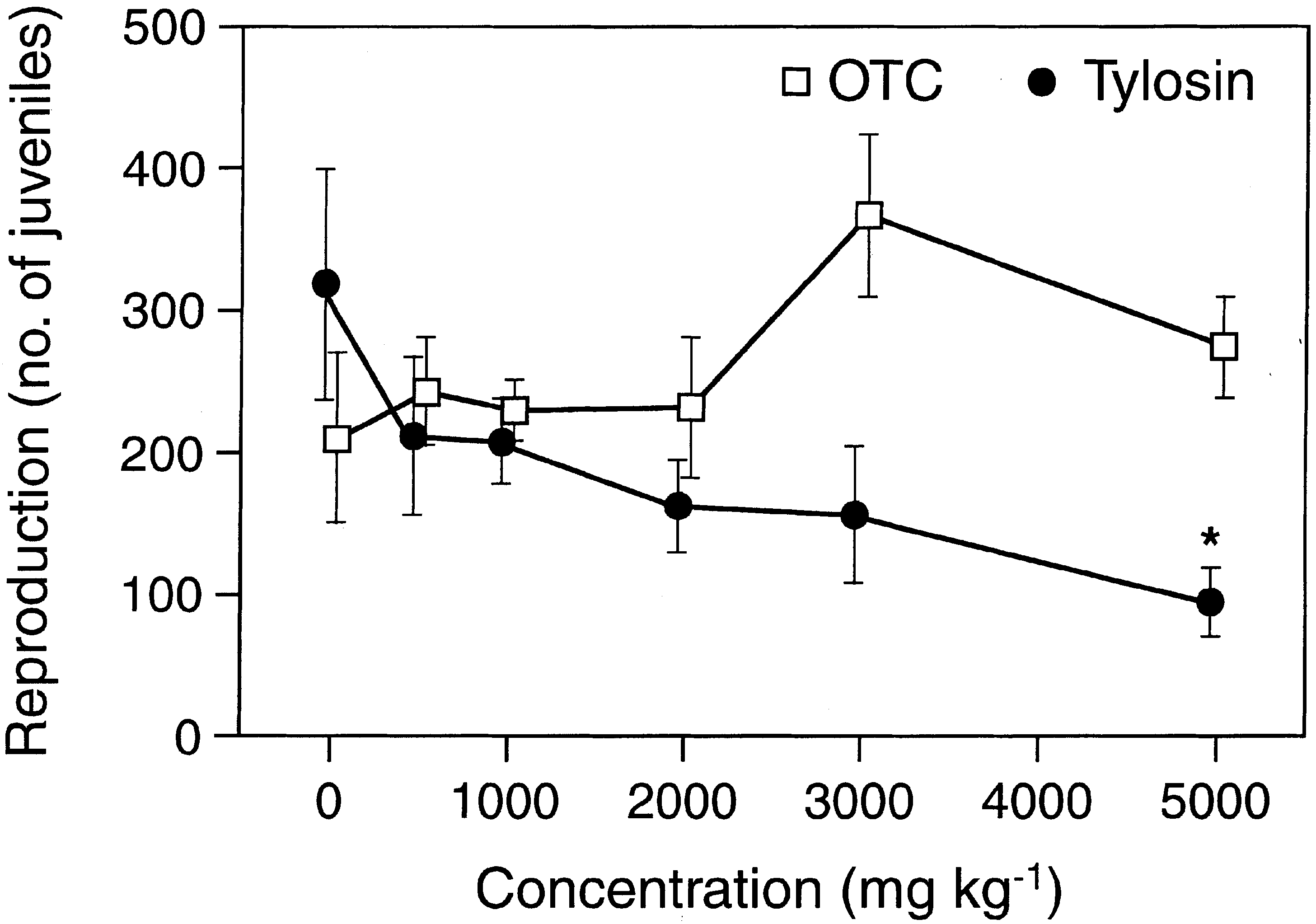
 A.J. Baguer et al. / Chemosphere 40 (2000) 751±757
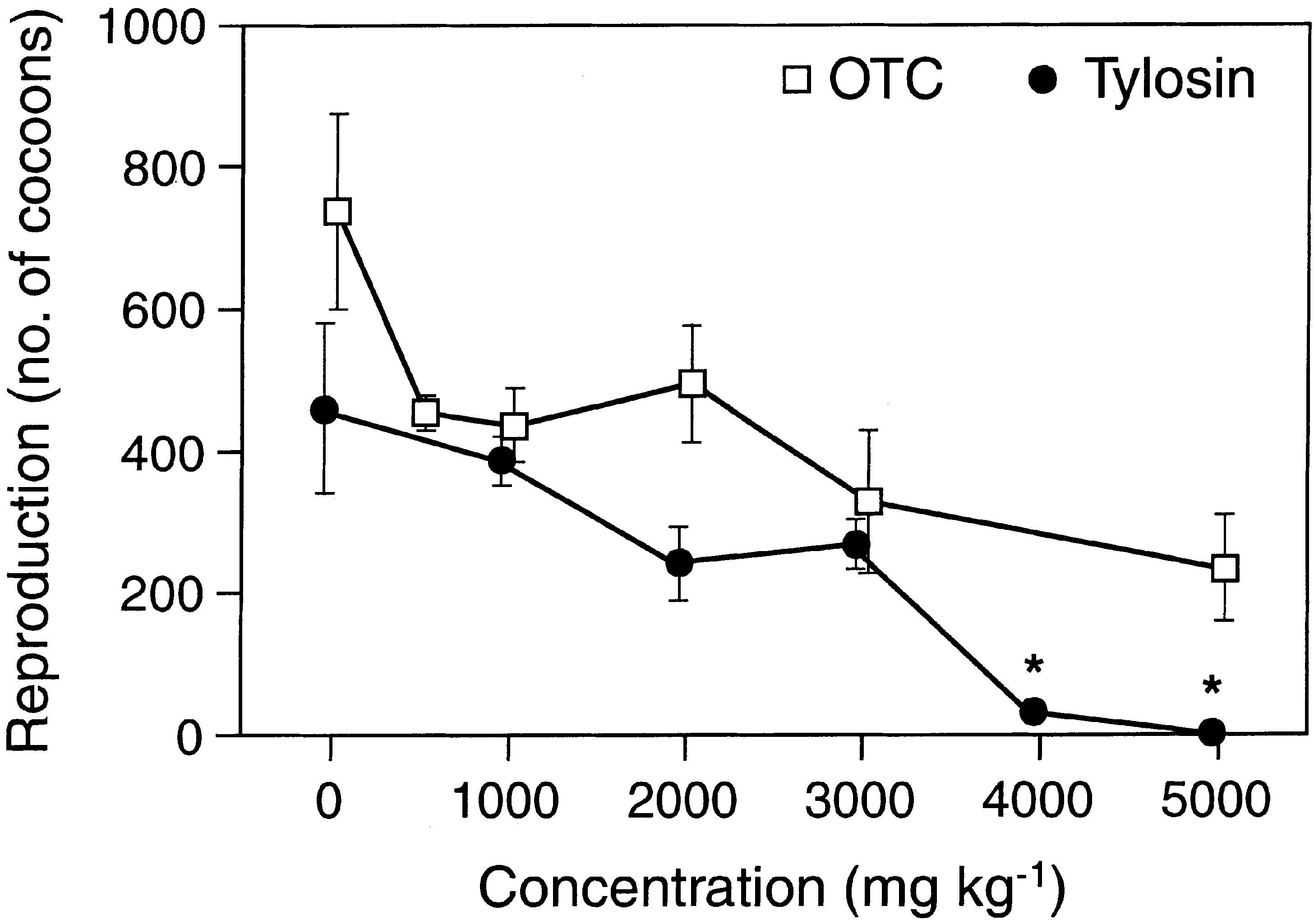
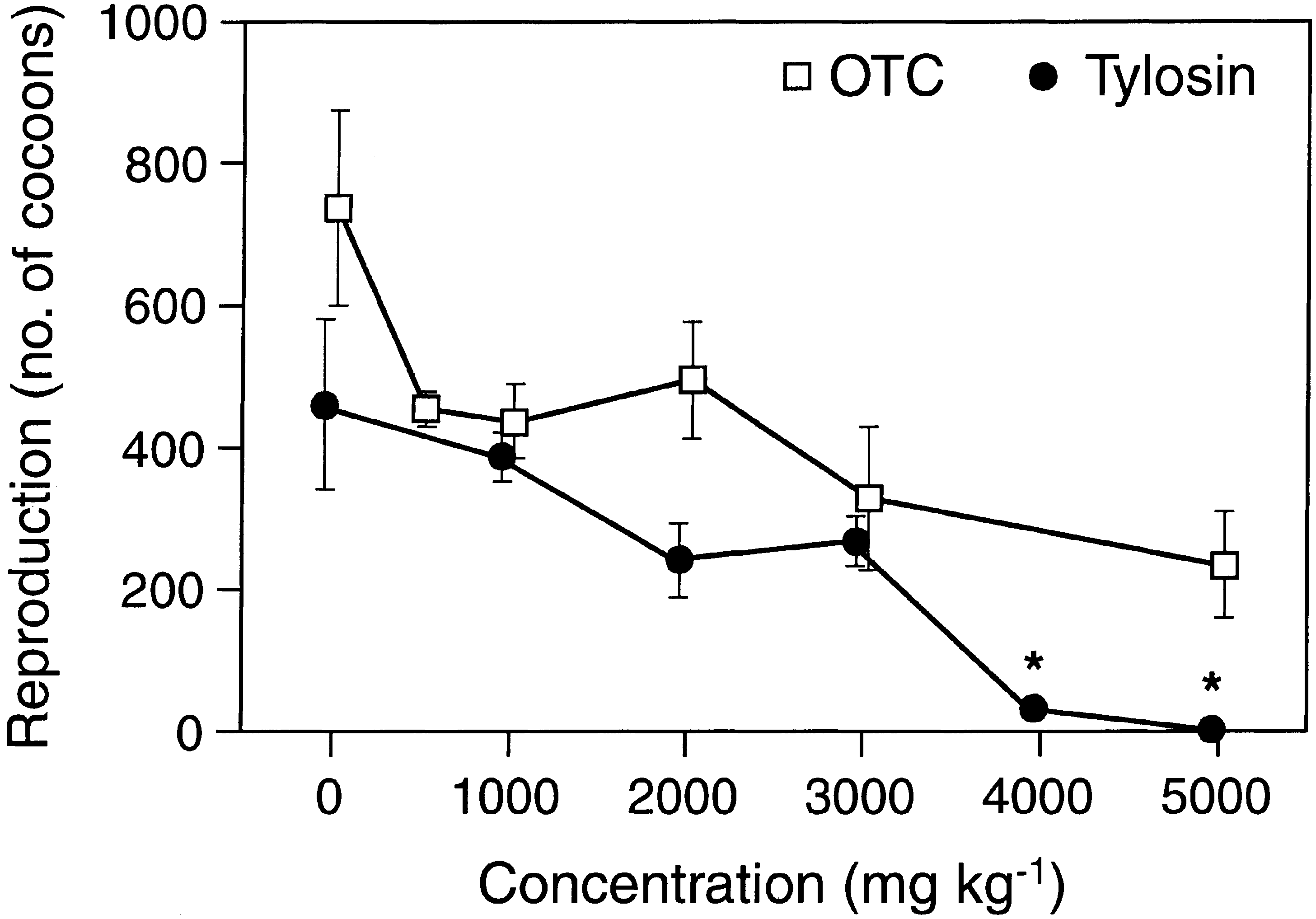
The toxicity of OTC and tylosine to the three tested
soil animals was generally very low. The lowest observed
signi®cant eects were found at 3000 mg kgÀ1 and in
many cases no eects were observed at the highest test
concentration of 5000 mg kgÀ1 (Figs. 1±3). Reproduc-
tion was generally a more sensitive endpoint than sur-
vival (Tables 1±4). Growth and fertility, expressed as the
number of cocoons hatched during a nine weeks post
exposure period, were a slightly more sensitive endpoints
than survival of earthworms. Estimated EC10 values
were found in the range of 134 to more than 5000 mg
kgÀ1, whereas all EC50 values were above 2000 mg kgÀ1.
A.J. Baguer et al. / Chemosphere 40 (2000) 751±757
The toxicity of OTC and tylosine to the three tested
soil animals was generally very low. The lowest observed
signi®cant eects were found at 3000 mg kgÀ1 and in
many cases no eects were observed at the highest test
concentration of 5000 mg kgÀ1 (Figs. 1±3). Reproduc-
tion was generally a more sensitive endpoint than sur-
vival (Tables 1±4). Growth and fertility, expressed as the
number of cocoons hatched during a nine weeks post
exposure period, were a slightly more sensitive endpoints
than survival of earthworms. Estimated EC10 values
were found in the range of 134 to more than 5000 mg
kgÀ1, whereas all EC50 values were above 2000 mg kgÀ1.