Unknown
Package Leaflet: Information for the User Read all of this leaflet carefully before 150 mg prolonged release you start using this medicine because it film-coated tablets contains important information for you. bupropion hydrochloride Keep this leaflet. You may need to read it again. If you have any further questions, ask your doctor or pharmacist. This medicine has be
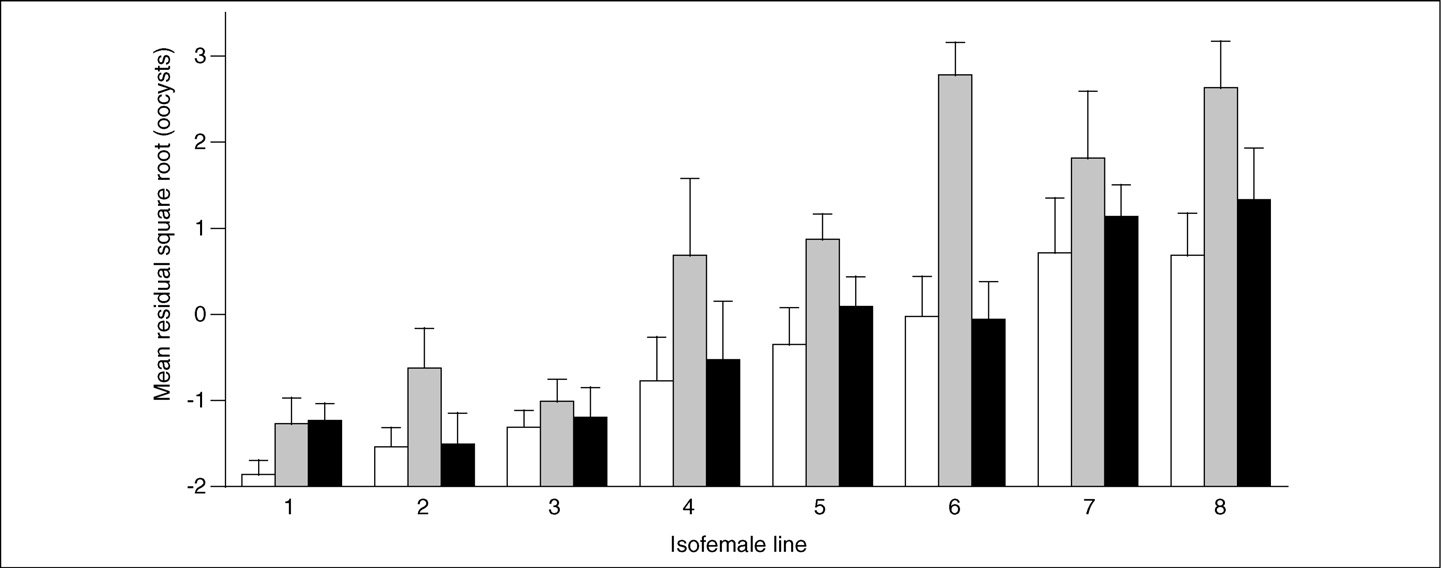 Figure 1. Genetic and environmental components of infection load. The mean number of oocysts (plus standard error) in infected mosquitoes is given for eight isofemalelines that were fed on 2% (white bars), 4% (grey bars) or 6% (black bars) glucose solutions. The lines are ranked along the x-axis according to their mean number of oocysts(averaged across glucose concentrations). The number of oocysts has been transformed using the square root and corrected with regards to the mice that were used to feedthe mosquitoes (the residual gives the difference from the average for a given mouse). Reproduced, with permission, from Ref.
sources , these sugar meals might not be important
outside enclosure) situation but also to do so with natural
to mosquito populations in the field that have regular
populations of mosquitoes and Plasmodium. Furthermore,
it is possible that findings from field studies will only be
There is clearly a need to investigate interactions
relevant for the particular area of study. In the wild,
between resistance genotypes and other environmental
different populations encounter different environmental
factors that could be as, or more, important in the field as
influences – could they also respond differently to them?
sugar meal concentrations. These could include tempera-ture fluctuations, which have been shown to impinge of the
background genetic basis of host resistance Even such
influences as the distance either from oviposition sites
environment matters for the functioning of a malaria-
or from future blood meals and the presence of potential
resistant phenotype in mosquitoes . They report an
predators will alter metabolic resources devoted to flight
important proof of principal that could have profound
and could, therefore, change the outcome of resistance to
effects on the dynamics and coevolution of this vector-
borne parasite. Resistance phenotypes are complex, evenin laboratory strains, and it remains to be seen whether
An environmental determinant of mortality
nurture has a substantial affect on malaria transmission in
There is still considerable controversy that surrounds the
effect of Plasmodium infection on mosquito mortality Lambrechts et al. provide firm evidence in support of the
negative impact of infection on mosquito fitness. All eight
1 Al-Mashhadani, H.M. et al. (1980) A genetic study of the susceptibility
isofemale lines, whatever their environmental regime,
of Anopheles gambiae to Plasmodium berghei. Trans. R. Soc. Trop.
Figure 1. Genetic and environmental components of infection load. The mean number of oocysts (plus standard error) in infected mosquitoes is given for eight isofemalelines that were fed on 2% (white bars), 4% (grey bars) or 6% (black bars) glucose solutions. The lines are ranked along the x-axis according to their mean number of oocysts(averaged across glucose concentrations). The number of oocysts has been transformed using the square root and corrected with regards to the mice that were used to feedthe mosquitoes (the residual gives the difference from the average for a given mouse). Reproduced, with permission, from Ref.
sources , these sugar meals might not be important
outside enclosure) situation but also to do so with natural
to mosquito populations in the field that have regular
populations of mosquitoes and Plasmodium. Furthermore,
it is possible that findings from field studies will only be
There is clearly a need to investigate interactions
relevant for the particular area of study. In the wild,
between resistance genotypes and other environmental
different populations encounter different environmental
factors that could be as, or more, important in the field as
influences – could they also respond differently to them?
sugar meal concentrations. These could include tempera-ture fluctuations, which have been shown to impinge of the
background genetic basis of host resistance Even such
influences as the distance either from oviposition sites
environment matters for the functioning of a malaria-
or from future blood meals and the presence of potential
resistant phenotype in mosquitoes . They report an
predators will alter metabolic resources devoted to flight
important proof of principal that could have profound
and could, therefore, change the outcome of resistance to
effects on the dynamics and coevolution of this vector-
borne parasite. Resistance phenotypes are complex, evenin laboratory strains, and it remains to be seen whether
An environmental determinant of mortality
nurture has a substantial affect on malaria transmission in
There is still considerable controversy that surrounds the
effect of Plasmodium infection on mosquito mortality Lambrechts et al. provide firm evidence in support of the
negative impact of infection on mosquito fitness. All eight
1 Al-Mashhadani, H.M. et al. (1980) A genetic study of the susceptibility
isofemale lines, whatever their environmental regime,
of Anopheles gambiae to Plasmodium berghei. Trans. R. Soc. Trop.