fi.ge.pgstatic.net
IIFT GK QUESTION BANK - 2011 Famous writer and Jnanpith Award recipient U.R. Ananthamurthy is short-listed for this year’s $50,000DSC Prize for South Asian Literature. He writes in whichThe Cabinet Committee on Economic Affairs (CCEA)recently has approved the setting up of how many newMega Food Park projects in addition to the 15 ongoingWhich among the following person has become the fir
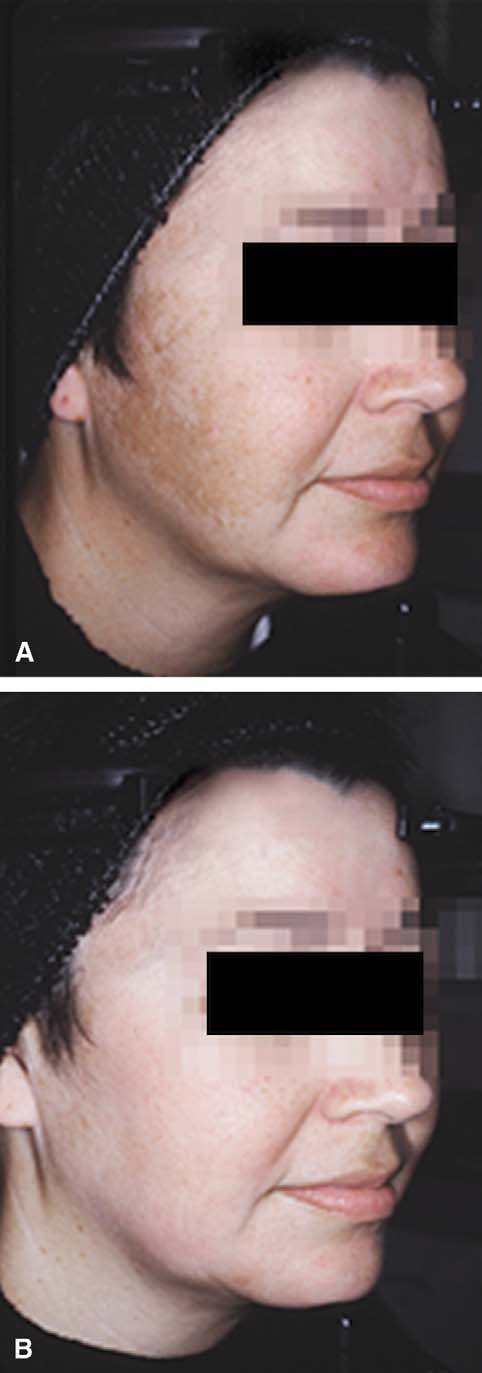 decreasing cellular metabolism. Tretinoin has beenfound to abrogate the epidermal atrophy that canoccur with topical corticosteroids. Various modifica-tions to the original formula have subsequently beenstudied.
decreasing cellular metabolism. Tretinoin has beenfound to abrogate the epidermal atrophy that canoccur with topical corticosteroids. Various modifica-tions to the original formula have subsequently beenstudied.