benefits.inventivhealth.com
High Deductible Health Plan (HDHP) - Health Savings Account (HSA) Preventive Therapy Drug List (10/01/11) ANTICONVULSANTS ORAL ANTIANGINAL AGENTS COMBINATION ANTIHYPERLIPIDEMICS DIABETES SL and chewable formulations are not included DIAGNOSTIC AGENTS TRANSDERMAL/TOPICAL ANTIANGINAL INJECTABLE DIABETES AGENTS CORONARY ARTERY DISEASE ANTIHYPERLIPIDE

 Journal of Organometallic Chemistry 558 (1998) 41 – 49
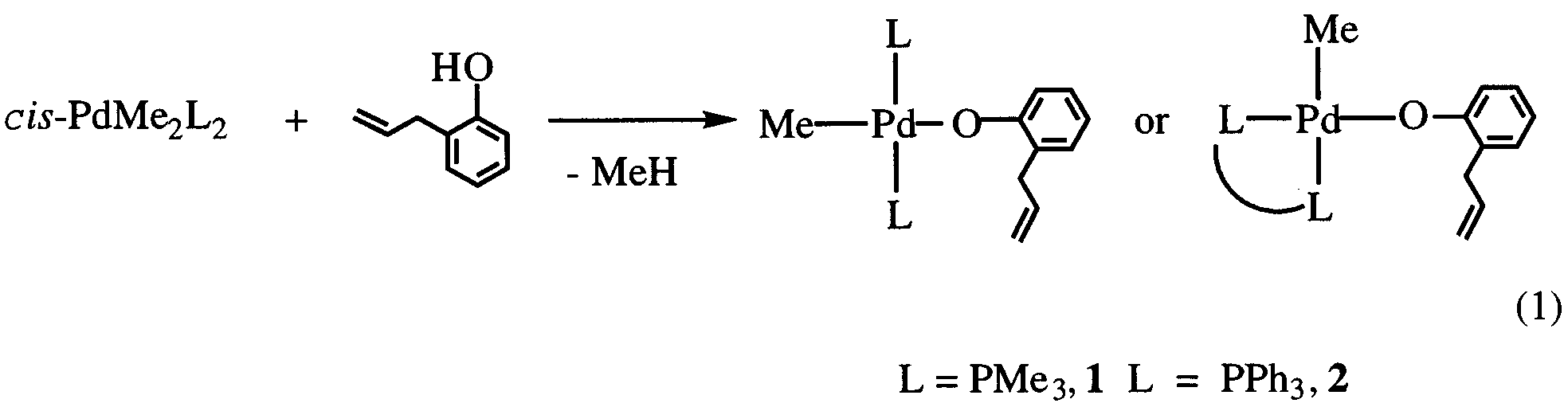
Synthesis and structure of methylpalladium(II) and -platinum(II)
trans-PdMe(O H CH CH CH -o)(PR ) (R
p2-C,C-OC H CH CH CH -o)(PMe ). Simple O-coordination and
chelating coordination depending on the metal center and auxiliary
Yong-Joo Kim a,*, Jae-Young Lee a, Kohtaro Osakada b
a Department of Chemistry, Kangnung National Uni6ersity, Kangnung, 210-702, South Korea
b Research Laboratory of Resources Utilization, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226, Japan
Received 8 July 1997; received in revised form 2 December 1997
Abstract
Journal of Organometallic Chemistry 558 (1998) 41 – 49
Synthesis and structure of methylpalladium(II) and -platinum(II)
trans-PdMe(O H CH CH CH -o)(PR ) (R
p2-C,C-OC H CH CH CH -o)(PMe ). Simple O-coordination and
chelating coordination depending on the metal center and auxiliary
Yong-Joo Kim a,*, Jae-Young Lee a, Kohtaro Osakada b
a Department of Chemistry, Kangnung National Uni6ersity, Kangnung, 210-702, South Korea
b Research Laboratory of Resources Utilization, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226, Japan
Received 8 July 1997; received in revised form 2 December 1997
Abstract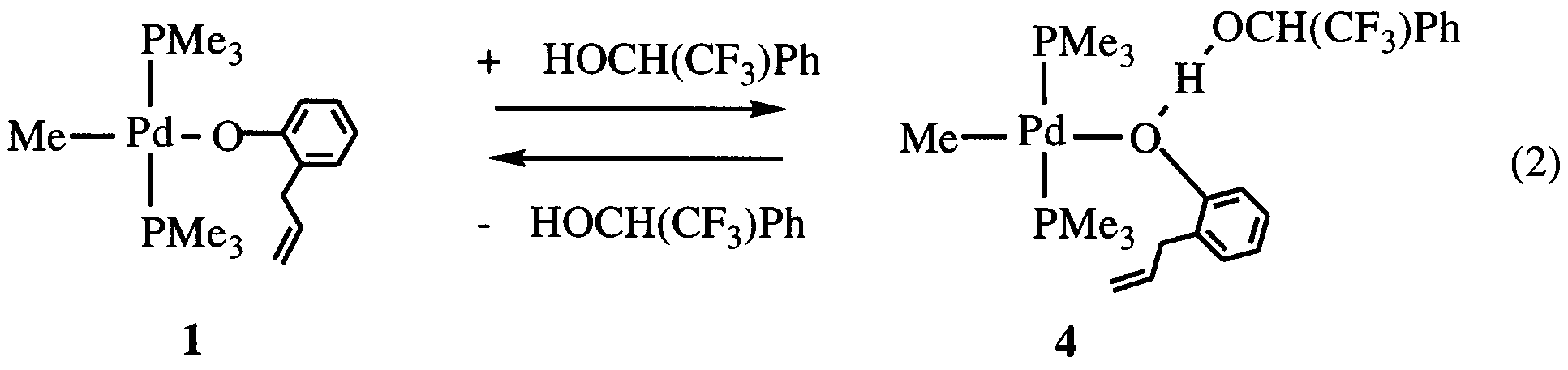
 Y.-J. Kim et al. / Journal of Organometallic Chemistry 558 (1998) 41 – 49
(II), -palladium (II), and -platinum (II) phenoxides or
caused by coupling with two unequivalent phosphorus
complexes, MR(OAr)L · (HOAr) containing phenol
nuclei. The 1H- and 13C-NMR signals of PMe ligands
associated with phenoxido ligands through hydrogen
of 1 and 13C{1H}-NMR peak due to ipso carbons of
Y.-J. Kim et al. / Journal of Organometallic Chemistry 558 (1998) 41 – 49
(II), -palladium (II), and -platinum (II) phenoxides or
caused by coupling with two unequivalent phosphorus
complexes, MR(OAr)L · (HOAr) containing phenol
nuclei. The 1H- and 13C-NMR signals of PMe ligands
associated with phenoxido ligands through hydrogen
of 1 and 13C{1H}-NMR peak due to ipso carbons of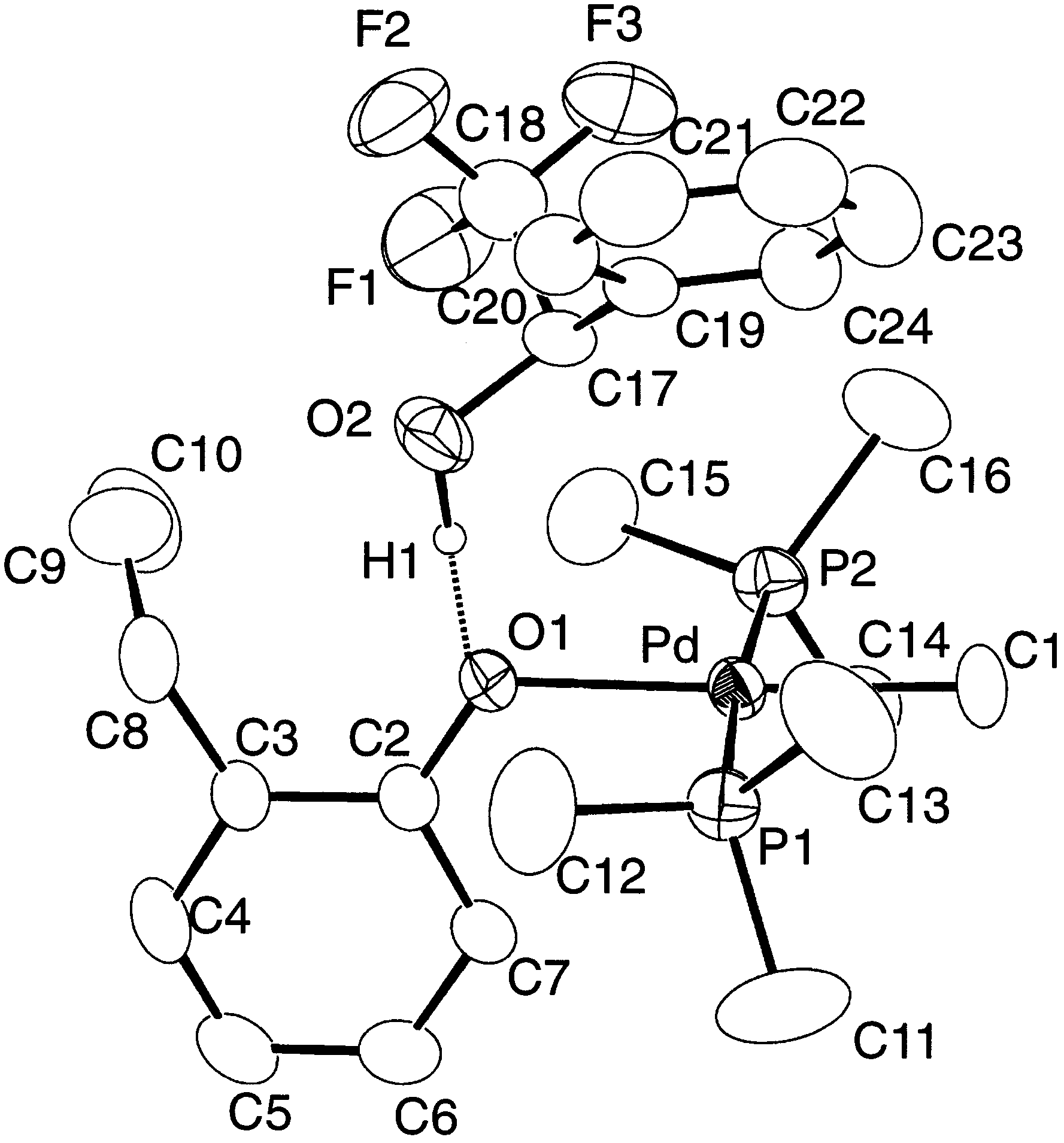
 Y.-J. Kim et al. / Journal of Organometallic Chemistry 558 (1998) 41 – 49
The 1H-NMR spectrum of 4 taken in CDCl (25°C)
Y.-J. Kim et al. / Journal of Organometallic Chemistry 558 (1998) 41 – 49
The 1H-NMR spectrum of 4 taken in CDCl (25°C)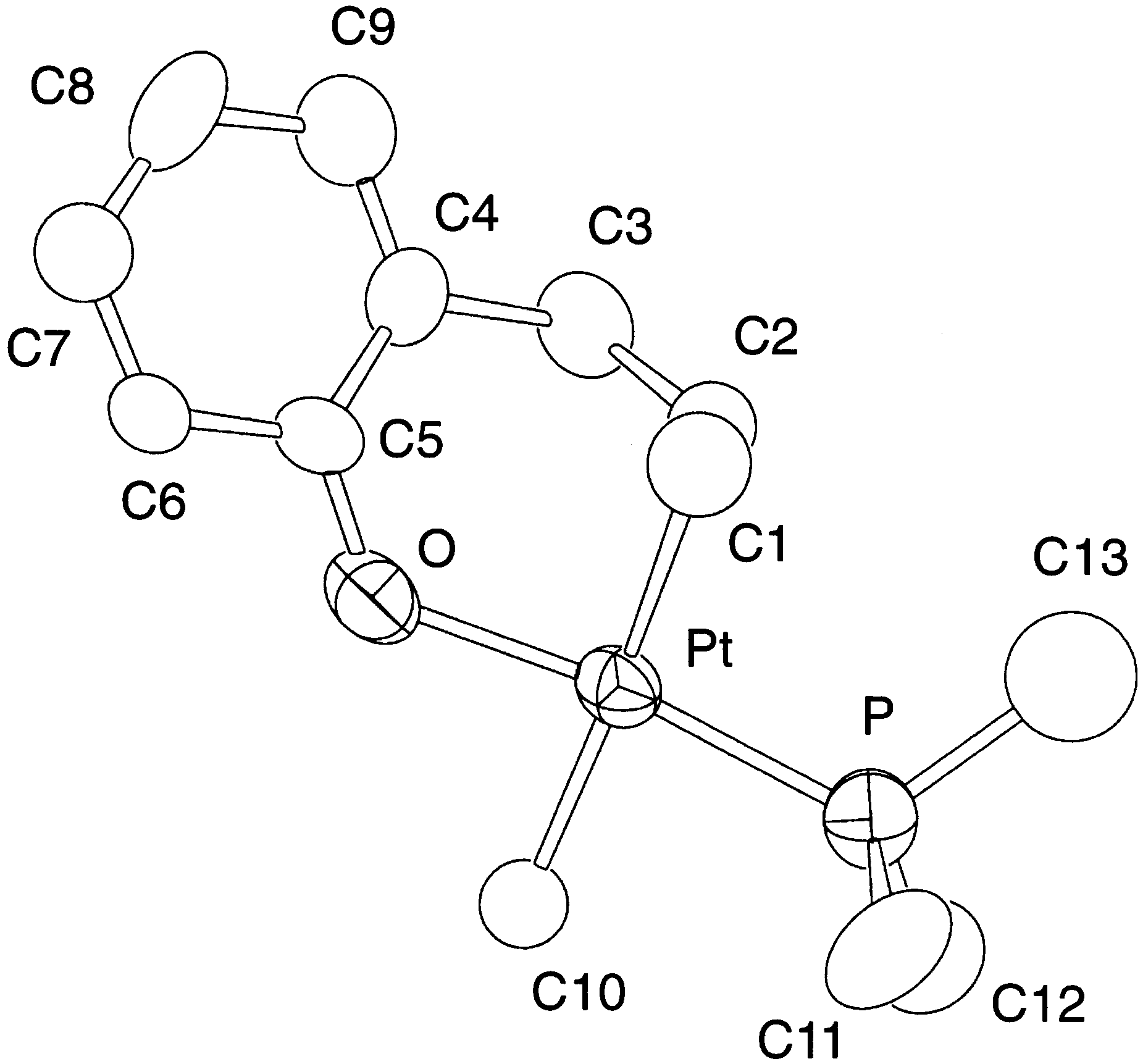
 Y.-J. Kim et al. / Journal of Organometallic Chemistry 558 (1998) 41 – 49
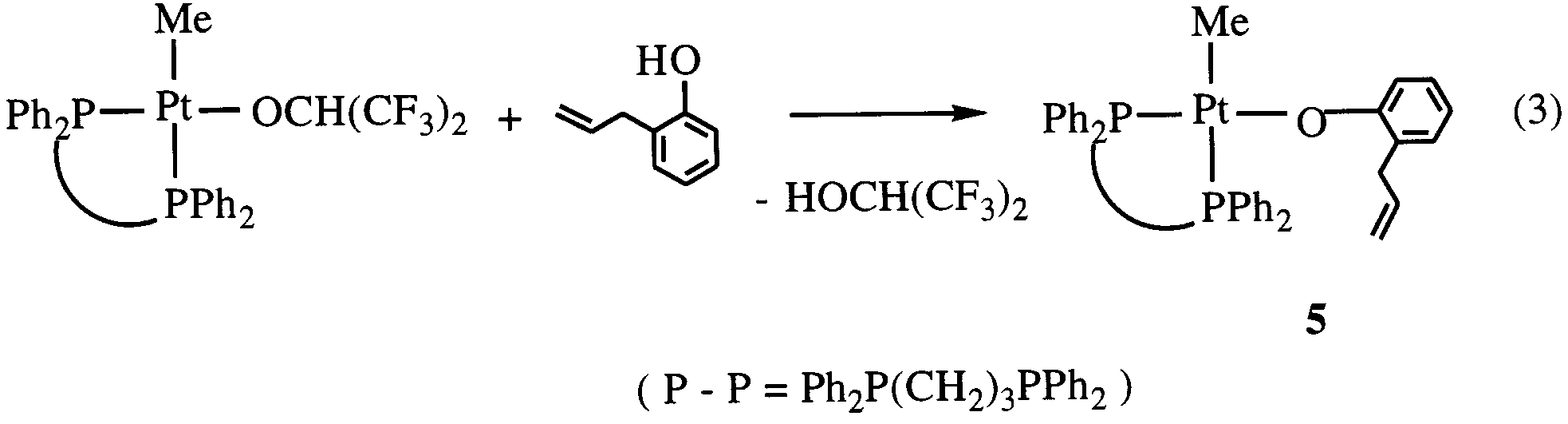
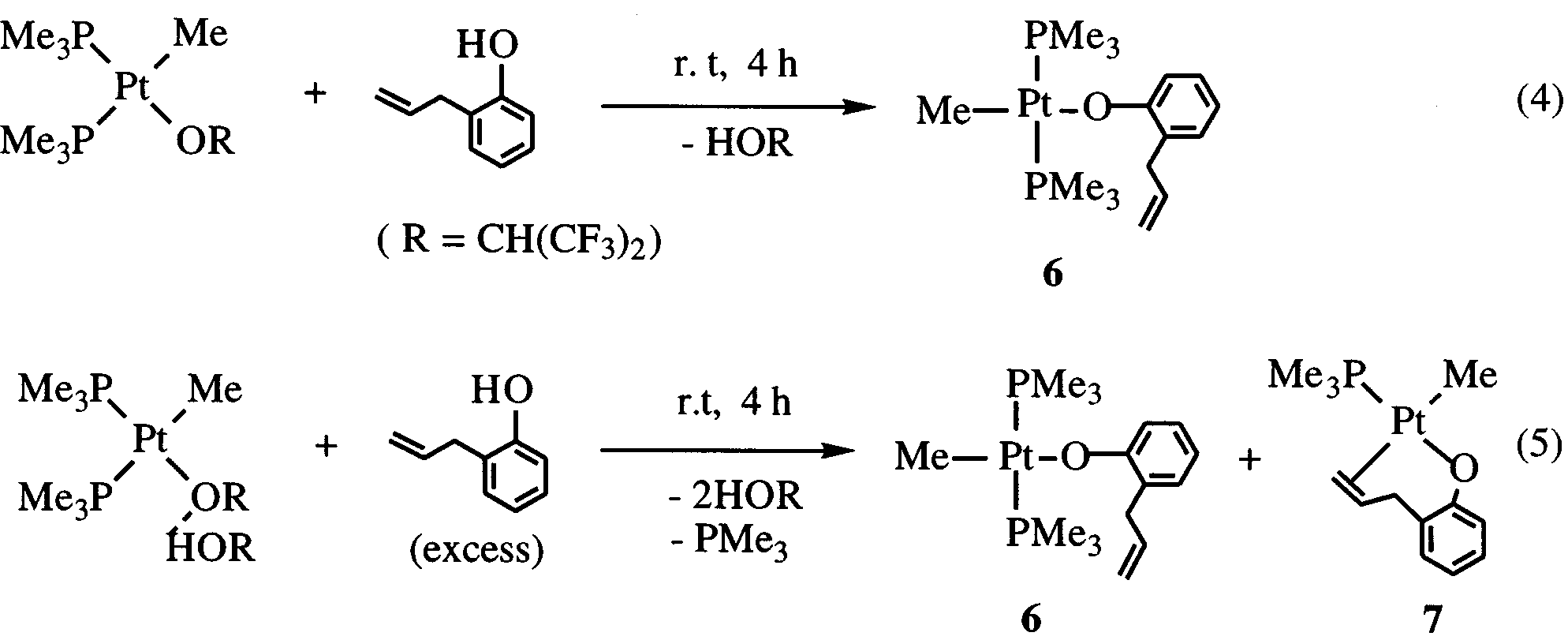
CH -o)(PMe ) (7) which are isolated by fractional
Y.-J. Kim et al. / Journal of Organometallic Chemistry 558 (1998) 41 – 49
CH -o)(PMe ) (7) which are isolated by fractional